With the increased number of people who have contracted coronavirus there is information that in some cases, the disease leaves behind some after effects. In particular Hong Kong’s Hospital Authority issued a release according to which 3 patients out of 12 ones under observation after COVID-19 infection have signs of a marked decrease in lung function: shortness of breath during brisk walking; in accordance with Dr. Tsang the lung function decline reaches 20-30% in these patients. Currently they are undergoing additional examinations. On the basis of preliminary data of patients at the Princess Margaret hospital certain changes in the lungs on computed tomography (CT) such as “ground-glass opacity” (GGO) are revealed. It indicates lesion of the organ. We are confident that more detailed information about patients who have recovered from COVID-19 associated pneumonia will soon be available. In addition Dr. Tsang plans to monitor long-term negative effects in recovered coronavirus patients such as the formation of pulmonary fibrosis.

How can mesenchymal stem cells (MSC) help in this situation?
During chronic or acute lung injury inadequate immune response and impaired restoration (repair) can cause irreversible lung damage which leads to the formation of fibrosis and reduced lung function. Therefore an active search is underway for new therapeutic strategies to reduce current inflammation and at the same time to stimulate the regeneration of damaged alveolar cells. MSC are able to modulate reproduction, activation and effector function of all cells of the immune system which play an important role in the pathogenesis of acute and chronic lung diseases. In addition to reducing the pro-inflammatory potential of immune cells which infiltrate the lung alveoli MSC stimulate regenerative processes in damaged tissue through paracrine mechanisms (using signaling molecules).
MSC and acute respiratory distress syndrome ( ARDS, en. - severe acute respiratory syndrome – SARS)
Currently the results of the first pilot study of intravenous infusion of umbilical cord mesenchymal stem cell (UC-MSC) in patients with pneumonia caused by COVID-19 have been published. It was conducted at Beijing YouAn Hospital in January-February 2020. 7 patients were included in this study and five of them had severe form. The results of this first and small study are encouraging. The pulmonary function and symptoms of these seven patients were significantly improved in 2 days after MSC transplantation and later all patients were recovered.
Among the potential mechanisms of this improvement the authors suggest the decrease of the overactivated cytokine-secreting immune cells CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells. They cause a reaction known as the cytokine storm phenomenon and it can lead to a major severe complication of coronavirus infection. It is an acute respiratory distress syndrome (ARDS eng. SARS that is a main reason of death in patients with COVID-19).
This assumption is supported by the fact that intravenous administration of MSC resulted in a significant decrease in the concentration of TNF-α (the key pro-inflammatory cytokine), and an increase in the content of IL-10 (the anti-inflammatory factor). Apart from that group of CD14+CD11c+CD11bmid regulatory DC cell population dramatically increased which has anti-inflammatory effect and also limits the intensity of inflammation in the lungs.
It should be noted that intravenous administration is the most effective way in the treatment of lung diseases because all the injected cells fall into the right parts of the heart and then into the pulmonary artery with the blood flow. The vast majority of injected MSC are concentrated in the pulmonary parenchyma within an hour after the infusion.
Of course it is necessary to continue research in the field of cell therapy in patients with COVID-19 on a larger number of patients. Currently it has been announced that several studies have begun in this direction.
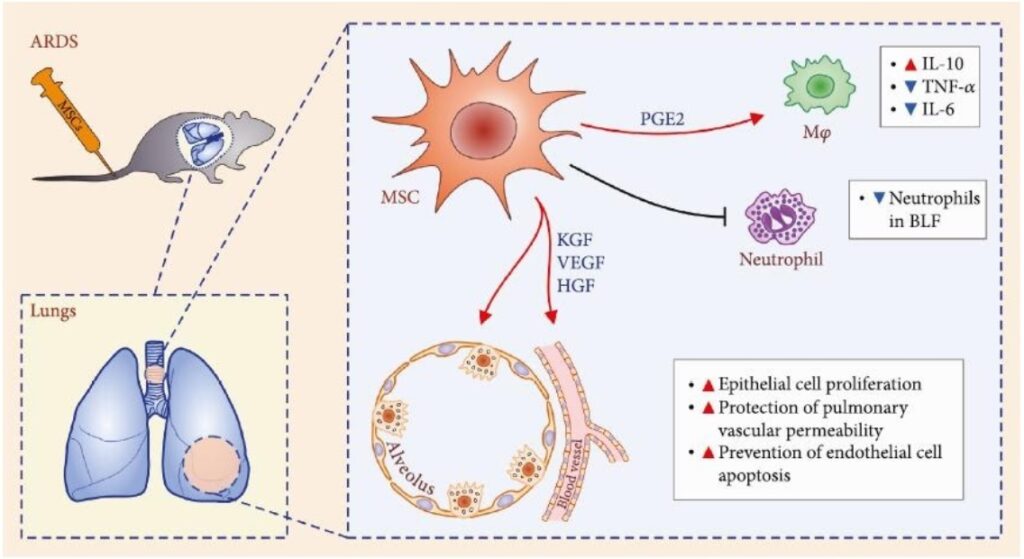
Recent preclinical studies shed more light on the role of MSC in ARDS therapy. Thus MSC reduce neutrophil accumulation in the lungs via prostaglandin E2 (PGE2) and interleukin 10 (IL-10) dependent mechanisms and decrease TNF-α production in ARDS induced by the administration of Escherichia coli lipopolysaccharide. Keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) produced by stem cells have been shown to stimulate the regeneration of type II alveolocytes, prevent endothelial cell death and restore the alveolar–capillary barrier in lungs damaged by ARDS? It leads to a decrease of local edema, improved oxygenation and survival.
The effects of intravenous administration of MSCs derived from bone marrow in people with ARDS have also been studied by Wilson and co-authors. The dosage of MSCs as 1.5 and 10 million /kg body weight was revealed to be safe and well tolerated by patients. The results were also optimistic and led to the organization of another large study. The outcome of it has not been published yet.
Thus the accumulated data allows us to classify the intravenous administration of mesenchymal stem cells as one of the promising methods of therapy for ARDS associated with COVID-19 disease.
MSC therapy of pulmonary fibrosis (PF) in COVID-19 convalescents
However with the growing number of COVID-19 patients with bilateral pneumonia which has been successfully treated the number of cases with severe post-inflammatory lung fibrosis will increase: phenomena reported by Dr. Tsang and Hong Kong’s Hospital Authority.
Currently there are no data even from pilot studies about the effects of mesenchymal stem cells in this cohort of patients but the study of the MSC role in the treatment of other conditions in which connective (fibrous, scar) tissue replaces normal parenchymal tissue allows us to expect optimistic official results.
Preclinical studies of mesenchymal stem cell (MSC) transplantation in chronic obstructive pulmonary disease (COPD) models with fibrosis and emphysema (main manifestations) have shown that one of the key targets for MSCs are macrophages: stem cells increased anti-inflammatory M2 macrophage markers and reduced pro-inflammatory cytokines of M1 macrophage as well MSCs reduced concentrations of MMP-2, MMP-9 and MMP-12 (matrix metalloproteinases) thereby preventing elastin degradation and the development of fibrosis in the lungs.
As a result the severity of emphysematous changes decreased, the loss of functioning alveoli slowed down and such parameters of ventilation as vital capacity (VC) and forced expiratory flow (FEF) improved. Predominantly mechanism of action via the paracrine effect is confirmed by the fact that the conditioned medium of the MSCs also has the listed positive effects.
Positive effects of MSCs in COPD are also associated with inhibition of apoptosis, i.e. programmed death of type 2 alveolocytes via increased expression of BCL-2 gene and inhibition of the caspase cascade which is a key mediator of apoptosis.
MSCs are able to prevent the development of lung fibrosis caused by radiation. The decrease of inflammatory damage to lung tissue in this study correlated with a decrease of cytokine production, fibroblast proliferation and collagen accumulation.
In the same way intravenous administration of MSCs protects lung against fibrosis caused by the toxin bleomycin: stem cell therapy demonstrated a reduction of alveolar inflammation, a decrease of the activity MMP-2, MMP-9, and MMP-13 known as key triggers of fibrosis and, most notably, improvement of lung structure and increased survival in experimental animals.
It should be emphasized that the key anti-fibrotic effects of MSCs are realized mainly at the stage of scar tissue formation and are mediated by the impact on the mechanisms of inflammation and cell death as well as stimulating regeneration of damaged alveolar cells. Based on this we assume that the maximum preventive effect in patients who have suffered from pneumonia and ARDS caused by COVID-19 coronavirus infection should be expected when the therapy is conducted within 5 months after the disease.
Conclusion
Summarizing the information above we can make the following observations:
- mesenchymal stem cell therapy has shown its effectiveness in the development of pneumonia and acute respiratory distress syndrome( ARDS, SARS), including those caused by coronavirus COVID-19
- mesenchymal stem cell therapy is a promising strategy for preventing the development of post-inflammatory lung fibrosis in patients after COVID-19-associated pneumonia
- optimal timing for this type of therapy is up to 4-5 months after the disease
- mesenchymal stem cell therapy in patients with lung diseases is tolerated well and does not lead to the development of side effects.